
Note: ImmuView® is not cleared for sale in the US
The quick, straightforward test for S. pneumoniae
ImmuView® S. pneumoniae is a rapid urinary Antigen test for detecting presumed pneumococcal pneumonia or meningitis.

Note: ImmuView® is not cleared for sale in the US
ImmuView® S. pneumoniae is a rapid urinary Antigen test for detecting presumed pneumococcal pneumonia or meningitis.
Get results fast in 3 simple steps
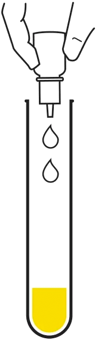
Add the sample to the test tube

Add the running buffer
and whirl gently
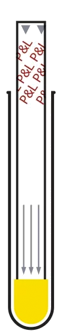
Insert the test and wait
just 15 minutes
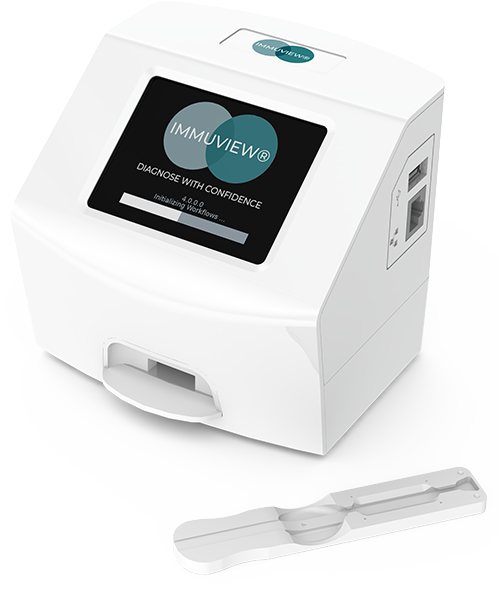
The ImmuView® Reader is a portable, highly flexible, rapid and easy to use test instrument.
Not only is it straightforward to use, but it quickly transfers your re¬sults through LIS/LIMs systems.
See the difference the reader makes
(Not cleared for sale in the US)
Yes
The ImmuView s.pneumoniae has not been validated for children under 8 years of age.
The “golden standard” for pneumococcus is blood culture.
We have not examined samples from children after vaccination, but we have looked at elderly (above 64 years) who have been vaccinated. Four (4 ) of 24 sample tested, were positive within the first 6 days. The 13-valent pneumococcal conjugate vaccine does not affect the ImmuView® S. pneumoniae Antigen Test. However, we do not recommend using the test within three days after vaccination with the 23-valent vaccine, as false-positive results might occur
It is a liquid positive control. The positive control should, when tested, give two lines
There is enough for at least three tests if the customer wants to use to three tests on positive control.
The kit does not require boiling
However, you can boil the sample to confirm a positive result, if the result is invalid, or if the urine sample contains visible blood.
Please refer to the IFU in the packaging for further explanation
Morning urine samples are preferred as the concentration of antigens will be highest in the morning. However, the test can easialy be used with tests collected throughout the day
The test has not been validated for use with synovial fluid. The test material validated is urine and CSF
The efficacy and safety of ImmuView® tests are fully supported by clinical data and documentation.
Urine samples
The clinical sensitivity and specificity were obtained by testing retrospective urine samples.
| ImmuView® S. pneumoniae Antigen Test |
IMMUVIEW® |
| Sensitivity (n=30) | 93% |
| Specificity (n=121) | 98% |
Cerebrospinal Fluid (CSF) Samples
Sensitivity was tested with culture positive samples and spiked samples. Specificity was tested with culture positive samples for other bacteria, culture negative and donor samples.
| ImmuView® S. pneumoniae Antigen Test |
IMMUVIEW® |
| Sensitivity (n=11) | 100% |
| Specificity (n=163)* | 98% |
* From the false positive samples, it was not possible to culture any bacteria from the samples which can be caused by too many times of freezing and thawing of the sample.
Store at room temperature. Expiry date is printed on the package.
| Article number | Product | Number of tests | Packing |
| 98748 | ImmuView® S. pneumoniae Antigen Test |
22 | 1 box |
You probably have a lot of questions about ImmuView®. We have the answers. And we’d love to show you how easily you can take your testing to a new level of convenience, speed and accuracy. So book a demo today.
Take your testing to new levels of convenience, speed and accuracy